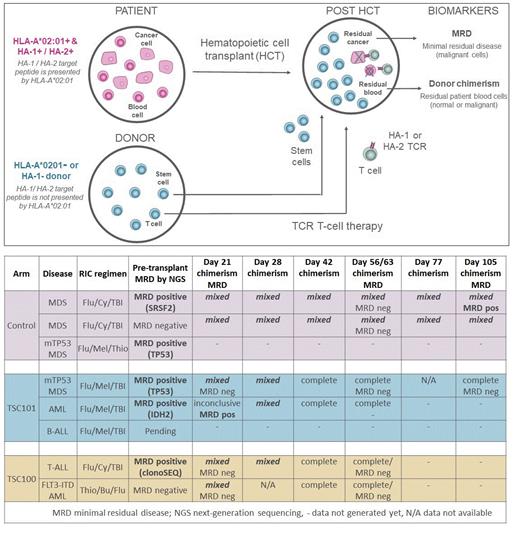
Introduction: To date, engineered T cell therapies have not proven effective for non-B cell hematologic malignancies due to a lack of antigens that spare healthy myeloid cells. Allogeneic hematopoietic cell transplantation (HCT) remains the best curative option for these malignancies, yet ~40% of patients relapse post-HCT with high mortality after relapse. A potential solution to prevent relapse is to target hematopoietic lineage-specific minor histocompatibility antigens (MiHAs) that are genetically mismatched between HCT patients and donors. These mismatches enable engineered T cells to selectively eliminate residual patient hematopoietic cells, normal or malignant, and spare donor cells. TSC-100 and TSC-101 are allogeneic, donor derived T-cell receptor-engineered T (TCR-T) cells that target MiHAs HA-1 and HA-2 respectively, both presented on HLA-A*02:01. By choosing HCT patients who are HLA-A*02:01 positive and donors who are either HLA-A*02:01 or MiHA negative, TSC-100 or TSC-101 are designed to eliminate residual patient hematopoietic cells post-HCT and thus prevent relapse (figure).
Methods: Study TSCAN-001 (NCT05473910) is a multi-center, multi-arm non-randomized, controlled Phase 1 study evaluating the feasibility, safety and preliminary efficacy of TSC-100 and TSC-101. Adults with AML, MDS or ALL eligible for reduced intensity conditioning (RIC)-based haploidentical donor transplantation from HLA or MiHA mismatched donors are enrolled. HLA-A*02:01 and HA-1 or HA-2-positive patients receive either TSC-100 or TSC-101 after HCT. HLA-A*02:01-negative control arm patients receive HCT alone. Upon count recovery after HCT, patients in treatment arms receive a single dose of TSC-100 or TSC-101 at Dose Level 1 or repeat doses at Dose Levels 2 and 3. Dose escalation follows interval 3+3 rules. Primary endpoints include adverse event profiles and dose-limiting toxicities (DLTs). Secondary endpoints are relapse rates, disease-free and overall survival. Exploratory endpoints include biomarkers of efficacy such as minimal residual disease (MRD) by deep next-generation sequencing (NGS), and donor chimerism using novel high-sensitivity and standard assays.
Results: At submission time, 8 patients were enrolled, 5 in treatment arms (3 TSC-101, 2 TSC-100) and 3 in the control arm, with a median of 111 days post-HCT follow-up (range 33-266 days). No DLTs occurred, and patients were enrolled at Dose Level 2 in both TSC-100 and TSC-101 arms. Safety analysis found expected post-HCT adverse events similar in treatment and control arms. Incidence of graft-versus-host disease (GvHD) was similar in control (3 events) and treatment arms (4 events). Serious adverse events of grade 3 GvHD and infections were observed in TSC-101 and control arms (1 event each). No cytokine release syndrome or neurotoxicity occurred after TSC-100/101 and minimal changes in CRP/ ferritin occurred, consistent with general safety of TCR-Ts with low target cell burden. No clinical relapses occurred to date.
Translational analysis found peak TSC-100/101 expansion and activation 7-14 days post dosing, with ongoing persistence at longest follow-up of 138 days. In repeat dose cohorts, substantial increases of early expansion were noted after dose 2 of TSC-101/100, supporting the benefit of repeat dosing. Chimerism analysis (table) using a novel high-sensitivity NGS-based AlloHeme assay (limit of detection (LOD) 0.13%) in whole blood, CD33+ or CD3+ subsets found mixed chimerism in 2 out of 2 control patients but complete donor chimerism in 4 out of 4 TSC-100/101 patients after Day 42. Both control patients had declining donor chimerism after Day 100 by AlloHeme and standard STR-based chimerism assays (LOD 1-2%) prompting early withdrawal of immunosuppression in one patient. Marrow MRD analysis by NGS (LOD <0.05%) found 1 control patient was MRD positive pre-HCT and remained MRD positive post-HCT. In contrast, 2 TSC-100/101 patients who were MRD positive pre-HCT turned MRD negative after HCT and TSC-100/101 therapy including a TP53 mutated MDS patient confirmed MRD negative by droplet digital PCR (LOD 0.01%).
Conclusions: Targeting MiHAs HA-1 or HA-2 with TSC-100/ 101 following HCT shows early safety and biomarker evidence of efficacy by completing elimination of all detectable patient hematopoietic cells, normal or malignant, thereby reducing relapse risk. Updated results will be presented at the meeting.
Disclosures
Al Malki:Tscan: Consultancy. Buonomo:TScan Therapeutics: Current Employment, Current equity holder in publicly-traded company. Wang:TScan Therapeutics: Current Employment, Current equity holder in publicly-traded company. Abelowitz:TScan Therapeutics: Current Employment, Current equity holder in publicly-traded company. Murray:TScan Therapeutics: Current Employment, Current equity holder in publicly-traded company. Macbeath:TScan Therapeutics: Current Employment, Current equity holder in publicly-traded company. Barton:TScan Therapeutics: Current Employment, Current equity holder in publicly-traded company. Chattopadhyay:TScan Therapeutics: Current Employment, Current equity holder in publicly-traded company. Reshef:TScan Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal